Volume 2 - Year 2016 - Pages 1-7
DOI: TBA
Third-Body Wear Behavior of Orthopedic Biopolymers
Binnur Sagbas1, M. Numan Durakbasa2
1Yildiz
Technical University, Department of Mechanical Engineering,
34349
Besiktas, Istanbul, Turkey
bsagbas@gmail.com
2Vienna
University of Technology,
Department
of Interchangeable Manufacturing and Industrial Metrology,
Karlsplatz
13/3113 A-1040 Wien, Austria
aum@mail.ift.tuwien.ac.at
Abstract - Third-body wear of orthopedic materials is very important parameter that affects the service life of artificial joints. Ultra High Molecular Weight Polyethylene (UHMWPE) has been the most preferred acetabular cup material for the past four decades. However wear is the primary problem waiting for to be solved. The wear debris of UHMWPE causes adverse tissue reactions and third-body wear damages which cause implant failure. For enhancement of UHMWPE tribological properties new materials such as vitamin E blended UHMWPE (VE-UHMWPE) have been developed for extending the implants life. Although many researches have been done about tribological behavior of conventional UHMWPE, there are limited numbers of study about third-body wear mechanism of vitamin E blended UHMWPE (VE-UHMWPE). The objective of this study is to determine the effect of PMMA bone cement as third-body particles on wear behavior of conventional UHMWPE and VE-UHMWPE. Pin-on-disc wear tests were applied under 60 N load and 3 hours in ultrapure water lubrication conditions. The results were evaluated for determining wear mechanism of disc materials.
Keywords: Biotribology, UHMWPE, Vitamin E, Third-body wear, Orthopedic implant.
© Copyright 2016 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2015-11-12
Date Accepted: 2016-01-04
Date Published: 2016-04-29
1. Introduction
Artificial total hip replacement is one of the most effective solutions for osteoarthritic natural joint which lost its functions. This replacement provides higher life quality to the patients by bringing back daily functions of the joint. Different material combinations such as metal-metal, metal-plastic, ceramic-ceramic, ceramic-plastic, have been used for femoral head and acetabular cup components of total hip joints [1].
Because of its excellent biocompatibility, impact load damping properties, chemical stability and low friction coefficient UHMWPE has been the most preferred acetabular cup material for the past four decades. However wear is the primary problem that limits service life of orthopaedic implants. The wear debris generated during articulation of joint materials could cause adverse tissue reactions, aseptic loosening, osteolysis and at the end implant loss. So, osteolysis and related aseptic loosening are significant problem limiting the lifetime of artificial hip and knee joints. Understanding the biotribological characteristics of the biomaterials is crucial for development of future implants [2],[3],[4],[5],[6],[7],[8],[9]. Research has been done for enhancement of UHMWPE properties such as low friction coefficient, third-body wear resistance, generation of small amounts of wear debris, and low cellular reactions to wear debris [10]. These enhancements are important for extending the implants life, especially for young and more active patients [11]. With the modification of the UHMWPE microstructure by radiation-induced cross-linking, and various thermal treatments, first generation cross-linked UHMWPEs have been developed [12],[13],[14]. Radiation cross-linked UHMWPE has shown higher wear resistance than that of conventional UHMWPE but mechanical properties such as ultimate tensile strength, yield strength and oxidation resistance have decreased [4],[15],[16],[17]. As a result of this delamination problem commonly occurs and it accelerates wear of prosthesis [18]. Also oxidation of UHMWPE causes decreasing of abrasive wear resistance of the material [19],[20]. For eliminating these negations second generation cross-linked UHMWPEs have been introduced which have been obtained by adding α-tocopherol or vitamin E, as a natural antioxidant, into UHMWPE to reduce the problems caused by the post irradiation thermal treatment [12]. In previous studies it was reported that the addition of vitamin E increases oxidation and delamination resistance of UHMWPE while maintaining the mechanical properties by stabilizing the residual free radical with eliminating post-irradiation melting process [19],[21],[22],[23].
Literature works about retrieved prosthesis show that third-body wear is a very important parameter affecting the service life of artificial joints [24]. By scratching the metal femoral head and femoral component of total hip and knee prosthesis third body particles promote the wear rate of UHMWPE acetabular cup and tibial component. PMMA bone cement particles are believed to be the main cause of third-body particles [25]. Besides, bone particles, metal beads or fibers from porous coatings and hydroxyapatite coatings, corrosion products from the metal tapers and metal fragments from other fixation devices may be the source of third body particles [26],[27],[28],[29],[30].
Although many researches have been done about tribological behavior of conventional UHMWPE, there are limited numbers of study about third-body wear mechanism of vitamin E blended UHMWPE (VE-UHMWPE). The objective of this study is to determine the effect of PMMA bone cement as third-body particles on wear behavior of conventional UHMWPE and VE-UHMWPE in ultrapure water lubrication conditions and comparing the results in terms of disc materials.
2. Materials and methods
UHMWPE and VE-UHMWPE disc samples were machined from Chirulen 1020 and Chirulen 1020 E rods (MediTECH Medical Polymers, Vreden, Germany) in 40 mm diameter and 5 mm thickness in accordance with ASTM G99-05 [31]. CoCrMo pin samples were used as counter face. Mechanical properties of UHMWPE, VE-UHMWPE and CoCrMo materials can be seen in table 1. Surface roughness of the samples was measured by Taylor Hobson Form Talysurf Intra. Average surface roughness of UHMWPE samples was 0.678 µm, of VE-UHMWPE was 0.653 µm. Pin-on-disc tribotester was used for wear tests and friction coefficient measurements. 60 N static load was applied with the frequency of motion 1 Hz and the tests were run up to 3 h. The tests were conducted in ultrapure water lubrication conditions.
Table 1. Mechanical properties of UHMWPE, VE-UHMWPE and CoCrMo.
The disk samples were cleaned in an ultrasonic bath, at 30oC, 15 min. in ultrapure water than 30 min. in ethyl alcohol at the end 15 min. ultrapure water respectively.
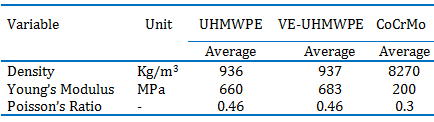
CoCrMo pin samples were manufactured in 5 mm diameter and 12 mm length. The tips of the pins were rounded in 5 mm diameter for obtaining higher contact pressure and homogeneous distribution of this pressure. Tip surface of the pins were polished by using 800, 1000, 1200 and 2000 grid sand papers. The average surface roughness of the samples were about 0,5 μm. The pins were cleaned in an ultrasonic bath. They were cleaned 15 min. in ultrapure water, 30 min. in acetone and 15 min. ultrapure water, respectively. Drawing of manufactured pin samples can be seen in Figure 1.
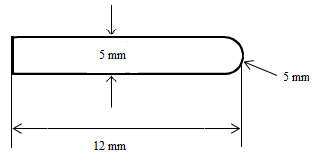
The diameters of the PMMA bone cement particles were measured by a particle size analyzer Malvern Nano ZS10 Zeta Sizer Nano Series (Malvern Instruments). The diameter of the PMMA particles changes between 264.3 nm and 412.5 nm and the average size of the diameters was about 339 nm. The morphological image of the PMMA particles, taken by Leica DCM 3D microscope, can be seen in Figure 2. As can be seen in this figure, the particles have smooth spherical shape. These particles were mixed with ultrapure water by using magnetic stirrer for preparing the wear test lubricant and the concentration of the solution was 10 g/L. Ultrapure water without PMMA particles was used for control.
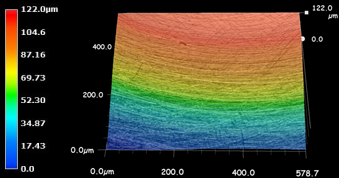
Wear track profile area was measured by Dektak 6 M Stylus Profiler for determining wear amount of the disc surfaces (Figure 3 and Figure 4). After wear tests the worn surfaces were analyzed by Keyence VHX Digital Microscope.
By using cross-sectional area of wear track and its radius the wear volume was calculated. Then by using Eq. (1) wear factor (k) of each disc sample was determined.
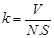
k; wear factor (mm3/N.m), V; wear volume (mm3), N; applied load (N), S; friction distance (m) [32],[33].
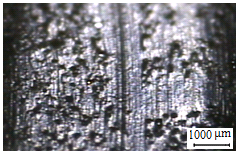 (a)
(a)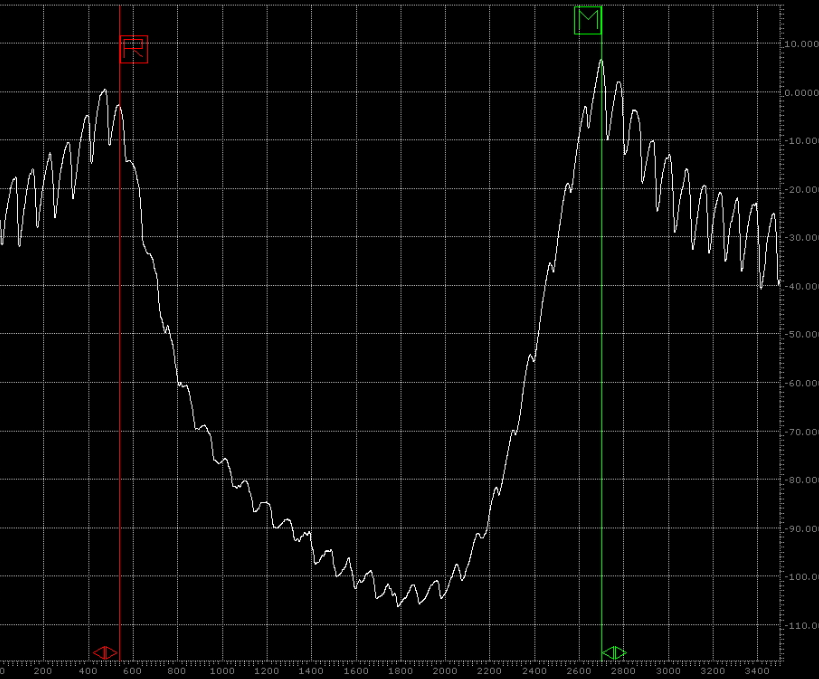 (b)
(b)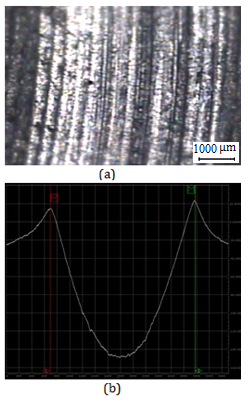
3. Results and Discussion
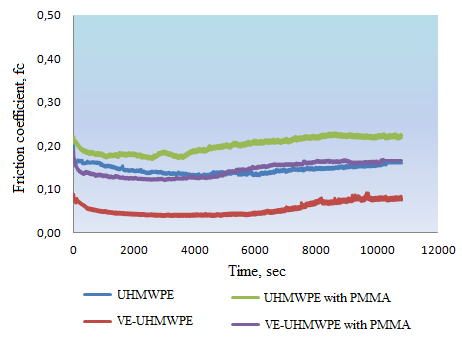
Friction coefficient of the pin-on-disc wear tests can be seen in Figure 5. Higher friction coefficients were recorded for each sample at the beginning of the tests because of the initial surface roughness of polymer component of the mating surfaces. After first 100-200 cycles the surfaces would be smoother than initial, so the friction coefficient values decreased. Because of the interacting mechanisms of PMMA particles between sliding surfaces, some deviations such as increases and decreases can be seen in the friction coefficient diagrams of the related samples. The friction coefficients increased with worn and scratched surfaces at the end of the tests. Wear factor k for UHMWPE was 3.28x10-5 mm3/N.m, for UHMWPE samples with PMMA third-body particles k became 4.90x10-5 mm3/N.m. For VE-UHMWPE disc samples without PMMA particles the wear factor was 3.00x10-5 mm3/N.m and for VE-UHMWPE with PMMA particles k was 3.35x10-5 mm3/N.m. Friction coefficient and wear factor of UHMWPE disc samples were higher than VE-UHMWPE samples' value in just ultrapure water lubrication condition without third-body abrasive particles. Likely, while PMMA abrasive particles were added in lubricant, friction coefficient and wear factor of two material groups were increased. In literature research different wear factors of UHMWPE have been reported between ranges of 10-5 -10-8 mm3N-1m-1 for different counter faces such as stainless steel, ZrO2, Al2O3, Co-Cr-Mo. These values may vary according to test conditions, surface topographical properties of the samples and lubricants. For example water generally does not form adequate boundary lubrication so wear factor may be smaller than serum lubricated test [35,36]. The percentage of serum also affects the wear amount. In reference [37] it is reported that higher wear factor obtained when 90 % serum was used instead of 25% serum. That may be because of the protein precipitation.
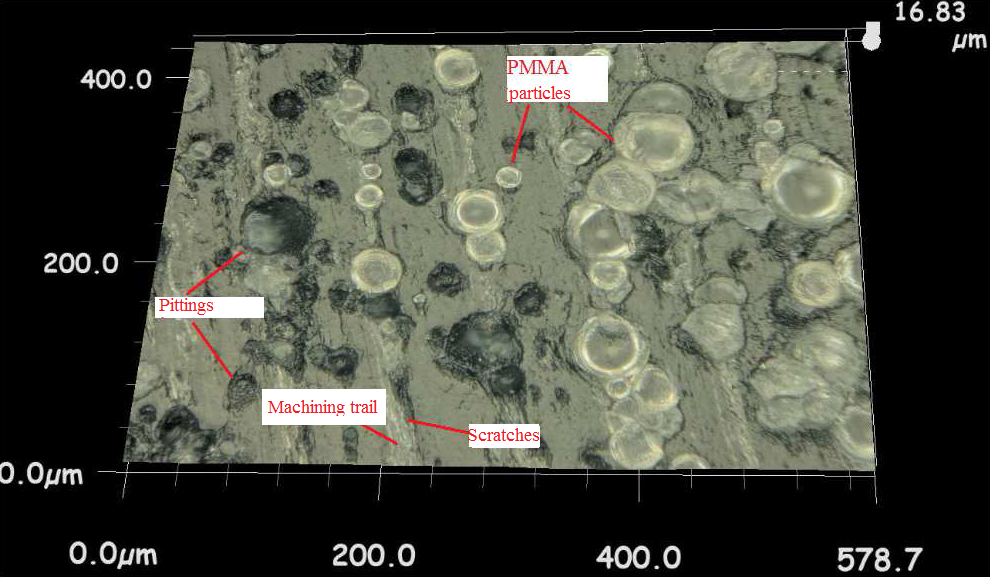
Three possible interacting mechanisms of PMMA particles with acetabular cup and femoral head sliding surfaces after being trapped at the interface were explained in a previous study [25]. First, particles may embed in polyethylene surface which cause to reduce the contact between the head and the cup. In second mechanism PMMA particles may adhere to the femoral head under pressure and lastly some particles may roll freely between the surfaces. In pin-on-disc configuration the embedded particles may cause pitting, the free particles that roll between surfaces may cause scratches on the UHMWPE disc surface as can be seen in Figure 6.
 (a)
(a)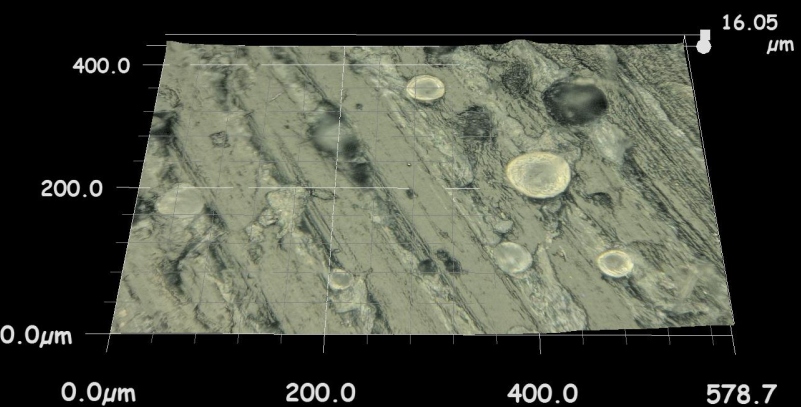 (b)
(b)Friction coefficient and wear factor of the VE-UHMWPE samples were lower than conventional UHMWPE. Sakoda et al., [38] studied with knee simulator for determining wear behavior of conventional UHMWPE and VE- UHMWPE knee prosthesis. They reported that, wear amount of VE-UHMWPE was 30% lower than of conventional UHMWPE. Addition of vitamin E increased the oxidation resistance of UHMWPE and decreased delamination and formation of surface cracks. So friction coefficient and wear factor of VE blended UHMWPE decreased. Similarly the third-body wear amounts of VE-UHMWPE samples were 9.3 % lower than conventional UHMWPE. As can be seen in Figure 6 amount of embedded PMMA third-body particles on conventional UHMWPE disc surface were more than on surface of VE-UHMWPE. It can be thought that vitamin E may increase the surface properties of UHMWPE.
 (a)
(a)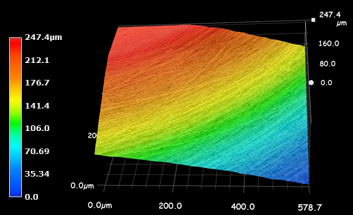 (b)
(b)Microscopic images of pin surface after wear tests can be seen in Figure 7. Scratches were visible on pin surface which tested in PMMA third-body lubrication condition. There were fewer scratches on without PMMA testing condition pin surface.
4. Conclusion
Although new materials have been developed for enhancement of UHMWPE tribological properties, wear is the primary factor that affecting the service life of implant. The wear debris of UHMWPE causes adverse tissue reactions and third-body wear damages which cause implant failure. For determining third-body wear mechanism of second generation UHMWPE, pin-on-disc wear tests were done. The results show that wear factor and friction coefficient of VE-UHMWPE was smaller than conventional UHMWPE. PMMA particles used as third-body abrasive material increased the wear rate of both material groups. Surface properties of VE-UHMWPE were better than conventional UHMWPE. The amount of embedded PMMA third-body particles on conventional UHMWPE disc surface were more than on surface of VE-UHMWPE. It can be thought that vitamin E may increase the surface quality of UHMWPE.
Acknowledgments
This research has been supported by Yildiz Technical University Scientific Research Projects Coordination Department. Project number: 2011-06-01-DOP03.The authors would like to thank Interchangeable Manufacturing and Industrial Metrology and Nanometrology Laboratory at the Vienna University of Technology, Wien, Austria, for support of surface measurements.
References
[1] F. Di Puccio and L. Mattei, "Biotribology of artificial hip joints," World Journal of Orthopedics, vol. 6, no. 1, pp. 77-94, 2015. View Article
[2] G. Bergmann, F. Graichen, A. Rohlmann, N. Verdonschot and G. H. Van Lenthe, "Frictional heating of total hip implants Part 2: Finite element study," Journal of Biomechanics, vol. 34, pp. 429-435, 2001. View Article
[3] M. Rocchi, S. Affatato and G. V. M. Falasca, "Thermomechanical analysis of ultra-high molecular weight polyethylene-metal hip prostheses," in Proceedings of the Institution of Mechanical Engineers Part H Journal of Engineering in Medicine, 2007. View Article
[4] G. N. Dong, M. Hua, J. Li and K. B. Chuah, "Temperature field and wear prediction for UHMWPE acetabular cup with assumed rectangular surface texture," Materials and Design, vol. 28, pp. 2402-2416, 2007. View Article
[5] J. E. Dowd, C. J. Sychterz, A. M. Young and C. A. Engh, "Characterization of long-term femoral-head-penetration rates," Association with and prediction of osteolysis, Journal of Bone Joint Surgery-American, vol. 82-A, pp. 1102-1107, 2000. View Article
[6] J. H. Dumbleton, M. T. Manley and A. A. Edidin, "A literature review of the association between wear rate and osteolysis in total hip arthroplasty," Journal of Arthroplasty, vol. 17, pp. 649-61, 2002. View Article
[7] S. K. Sinha and B. J. Briscoe, Polymer Tribology. Imperial College Press, 2009. View Article
[8] H. Bhatt and T. Goswami, "Implant wear mechanisms-basic approach," Biomedical Materials, pp. 31-39, 2008. View Article
[9] D. Xiong and S. Ge, "Friction and wear properties of UHMWPE/Al2O3 ceramic under different lubricating conditions," Wear, vol. 250, pp. 242-245, 2011. View Article
[10] H. Minakawa, M. H. Stone, B. M. Wroblewski, J. G. Lancaster, E. Ingham and J. Fisher, "Quantification of third body damage and its effect on UHMWPE wear with different types of femoral head," Journal of Bone and Joint Surgery British, vol. 80, no. 5, pp. 894-902, 1998. View Article
[11] F. Renò and M. Cannas, "UHMWPE and vitamin E bioactivity: an emerging perspective," Biomaterials, vol. 27, no. 16, pp. 3039-3043, 2006. View Article
[12] C. Vaidya, E. Alvarez, J. Vinciguerra, D. A. Bruce and J. D. DesJardins, "Reduction of total knee replacement wear with vitamin E blended highly cross-linked ultra- high molecular weight polyethylene," in Proceedings of the Institution of Mechanical Engineers Part H: Journal of Engineering in Medicine, 2011. View Article
[13] J. L. Tipper, A. L. Galvin, S. Williams, H. M. J. McEwen, M. H. Stone, E. Ingham and J. Fisher, "Isolation and characterization of UHMWPE wear particles down to ten nanometers in size from in vitro hip and knee joint simulators," J. Biomed. Mater. Res., Part A, vol. 78 A, no. 3, pp. 473-480, 2006. View Article
[14] J. S. Bergstrom and J. E. Bischoff, "An Advanced Thermo mechanical Constitutive Model for UHMWPE," International Journal of Structural Changes in Solids- Mechanics and Applications, vol. 2, no. 1, pp. 31-39, 2010. View Article
[15] C. A. Jacobs, C. P. Christensen, A. S. Greenwald and H. McKellop, "Clinical Performance of Highly Cross-Linked Polyethylenes in Total Hip Arthroplasty," The Journal of Bone & Joint Surgery Am, vol. 89, pp. 2779-2786. View Article
[16] L. Bradford, D. A. Bbaker, J. Graham, A. Chawan, M. N. Ries and L. A. Pruitt, "Wear and Surface Cracking in Early Retrieved Highly Cross-Linked Polyethylene Acetabular Liners," Journal of Bone & Joint Surgery, vol. 86, no. 6, pp. 1271-1282, 2004. View Article
[17] J. Furmanski, S. Gupta, A. Chawan, A. Kohm, J. Lannutti, B. Jewett, L. A. Pruitt and M. D. Ries, "Aspherical femoral head with highly cross-linked ultra-high molecular weight polyethylene surface cracking," Journal of Bone & Joint Surgery, vol. 89, pp. 2266-2270, 2001. View Article
[18] B. R. Micheli, K. K. Wannomae, A. J. Lozynsky, S. D. Christensen and O. K. Muratoglu, "Knee simulator wear of vitamin E stabilized irradiated ultrahigh molecular weight polyethylene," The Journal of Arthroplasty, vol. 27, pp. 95-104, 2012. View Article
[19] P. Bracco and E. Oral, "Vitamin E-stabilized UHMWPE for Total Joint Implants," Clinical Orthopaedics and Related Research, vol. 469, pp. 2286-2293, 2011. View Article
[20] G. Blunn, E. M. Brach del Preva, L. Costa, J. Fisher and M. A. R. Freeman, "Ultra high Molecular-weight polyethylene (UHMWPE) in total knee replacement: fabrication, sterilisation and wear," Journal of Bone Joint Surgery British, vol. 84, pp. 946-949, 2002. View Article
[21] E. Oral, S. D. Christensen, A. S. Malhi, K. K. Wannomae and O. K. Muratoglu, "Wear Resistance and Mechanical Properties of Highly Cross-linked, Ultrahigh-Molecular Weight Polyethylene Doped With Vitamin E," Joint Arthroplasty, vol. 4, pp. 580-591, 2006. View Article
[22] E. Oral, S. L. Rowell and O. K. Muratoğlu, "The effect of a-tocopherol on the oxidation and free radical decay in irradiated uhmwpe," Biomaterials, vol. 27, no. 32, pp. 5580-5587, 2006. View Article
[23] E. Oral, K. K. Wannomae, N. Hawkins, W. H. Harris and O. K. Muratoglu, "Alpha- ocopherol-doped irradiated UHMWPE for high fatigue resistance and low wear," Biomaterials, vol. 25, no. 24, pp. 5515-22, 2004. View Article
[24] C. Bragdon, M. Jasty, O. K. Muratoglu, D. O. O'Connor and W. H. Harris, "Third-body wear of highly cross-linked polyethylene in a hip simulator," J. Arthroplasy, vol. 18, no. 5, pp. 553-561, 2003. View Article
[25] A. Wang and A. Essner, "Three-body wear of UHMWPE acetabular cups by PMMA particles against CoCr, alumina and zirconia heads in a hip joint simulator," Wear, vol. 250, pp. 212-216, 2001. View Article
[26] K. Hirakawa, J. J. Jacobs, R. Urban and T. Saito, "Mechanisms of failure of total hip replacements: lessons learned from retrieval studies," Clinical Orthopaedics and Related Research, vol. 420, pp. 10-17, 2004. View Article
[27] Y. H. Kim, A. Ritchie and C. Hardaker, "Surface roughness of ceramic femoral heads after in vivo transfer of metal: correlation to polyethylene wear," Journal of Bone and Joint Surgery, American, vol. 87, pp. 577-582, 2005. View Article
[28] D. Mackay, A. Gower, B. Mawhinney, P. J. Gregg and A. W. McCaskie, "Metallic instrument debris: a source of third-body wear particles?," Journal of Arthroplasty, vol. 15, pp. 816-818, 2000. View Article
[29] B. M. Willie, J. E. Shea, R. D. Bloebaum and A. A. Hofmann, "Elemental and morphological identification of third-body particulate and calcium stearate inclusions in polyethylene components," Journal of Biomedical Materials Research, vol. 53, pp. 137-142, 2000. View Article
[30] L. Que and L. D. Topoleski, "Third-body wear of cobalt-chromium- molybdenum implant alloys initiated by bone and poly(methyl methacrylate) particles," Journal of Biomedical Materials Research, vol. 50, pp. 322-330, 2000. View Article
[31] A. G99-05, "Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus," 2010. View Article
[32] L. Ma, V. M. Rainforth, D. Sun, J. A. Wharton and R. J. K. Wood, "A '3-body' abrasion wear study of bioceramics for total hip joint replacements," Wear, vol. 267, pp. 2122-2131, 2009. View Article
[33] T. Pylios and D. E. T. Shepherd, "Wear of Medical Grade Silicone Rubber Against titanium and Ultrahigh Molecular Weight Polyethylene," Inc. J Biomed Mater Res Part B: Appl. Biomater, vol. 84B, pp. 520-523, 2008. View Article
[34] B. Sagbas, "Measurement, analysis and metrological evaluation of physical magnitudes and geometrical features that show alteration with regard to friction in hip prosthesis," Ph.D. Thesis, Dept. Mechanical Eng., Yildiz Technical University, Istanbul, 2013.
[35] H. J. Cho, W. J. Wei, H. C. Kao and C. K. Cheng, "Wear behavior of UHMWPE sliding on artificial hip arthroplasty materials," Materials Chemistry and Physics, vol. 88, pp. 9-16, 2004. View Article
[36] R. Cowie, A. Briscoe, J. Fisher and L. Jennings, "Influence of Lubricant and Temperature on the Wear of UHMWPE Articulating Against PEEK Optima," The Bone and Joint Journal, vol. 98-B, pp. 100, 2016. View Article
[37] A. Wang, V. K. Polineni, C. Stark and J. H. Dumbleton, "Effect of Femoral Head Surface Roughness on the Wear of Ultrahigh Molecular Weight Polyethylene Acetabular Cups," The Journal of Arthroplasty, vol. 13-6, pp. 615-620, 1998. View Article
[38] H. Sakoda, D. Nono, K. Kuramoto, M. Suzuki, H. Moriya and N. Tomita, "Superior wear resistance of vitamin E added uhmwpe tested on knee joint simulator," in 52nd Annual Meeting of the Orthopaedic Research Society, Chicago, 2006. View Article

