Volume 4 - Year 2018 - Pages 1-5
DOI: 10.11159/ijmmme.2018.001
Accompanying Elements in Zinc Oxide Emitted into the Environment in Pyrometallurgical Process of Zinc and Lead Production
Zdzisław Adamczyk, Katarzyna Nowińska
Silesian University of Technology, Department of Mining and Geology
2 Akademicka, Gliwice, Poland, 44-100
zdzislaw.adamczyk@polsl.pl; katarzyna.nowinska@polsl.pl
Abstract - The paper presents results of investigations of the content of selected accompanying elements in identified zinc oxide inclusions in the charge mixture and dusts emitted from a sintering machine into the environment from the pyrometallurgical production process of zinc and lead. The average content of the elements determined, i.e. Si, Al, Mn, Mg, Ca, K, Ag, As, Cd, Cu, Se, Sb, Sn, shows large variation, both in the charge mixture and furnace dust, and statistically low coefficients of correlation with zinc oxide and identified phases that form admixtures in ZnO, i.e. FeO and PbO.
Keywords: Pyrometallurgy, Zinc, Lead, Imperial Smelting Process, Sintering Machine, Zinc oxide, Accompanying elements
© Copyright 2018 Authors This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2015-09-04
Date Accepted: 2017-12-05
Date Published: 2018-02-05
1. Introduction
The “Miasteczko Śląskie” Zinc Smelting Plant is the only zinc and lead manufacturer in Europe that uses the ISP (Imperial Smelting Process) pyrometallurgical process. The basic production departments of the smelting plant include the Sinter Unit and the Shaft Furnace Unit [1].
In the sintering machine at the Sinter Unit the charge mixture is blast roasted at 1250°C - 1350°C to form Zn-Pb sinter which is transferred to the Shaft Furnace Unit. The charge for the sintering machine comprises a blend of raw materials (Zn and Pb concentrates), intermediates (Zn-Pb sinter), products (metallurgical Zn, crude Pb) and waste products (dust, dross, recycles, slag). During the oxidation process in the sintering machine, dust (called roasting dust) is emitted [2].
Previous studies have revealed the presence of a number of accompanying elements in the raw materials and wastes used in the ISP process, among them: Si, Al, Mn, Mg, Ca, K, Ag, As, Cd, Cu, Se, Sb, Sn [3, 4].
The mineral composition of the charge mixture and the dusts listed above is diverse, with the main constituents including: sulphides (ZnS, PbS, FeS2), oxides (ZnO, PbO, FeO) and sulphates (Pb, Zn, Fe). These constituents rarely occur in the form of individual grains. The dust was also found to contain silicates and sulphates. Additionally, there are other phases – lead carbonate or cerussite (PbCO3), lead oxychloride or damaraite (Pb4O3Cl2), In most cases they form complex multiphase systems, being the result of phase transformations occurring in the course of the pyrometallurgical process [5, 6].
Study of the forms of occurrence of these elements in materials from the various process units helps in determining the optimum method of obtaining these elements. Moreover, the identification of the phase composition of dusts and slags enables determining the environmental impact of wastes from ISP.
The aim of this study was to determine the content of accompanying elements and demonstrate the diversity thereof in zinc oxide derived from the charge mixture and from roasting dusts generated during oxidizing sintering in the sintering machine for the pyrometallurgical production of zinc and lead.
These investigations are a continuation of previous studies of the phase composition of raw materials and dusts formed during the ISP process, both in terms of the main phases, as well as of accompanying elements.
Previous research allowed to determine the variability of the content of accompanying elements in galena and sphalerite grains contained in the charge mixture and dusts from ISP [7, 8].
2. Sampling and Testing Methods
Samples were taken from the charge mixture (MS-2) and from dust from textile filters (FT12 and FT 24) – samples PR2 and PR3, respectively, from the process line of the sintering machine.
Chemical composition of adequately prepared samples was determined by means of a Joel JCXA 733 X-ray microanalyser, equipped with an ISIS 300 wavelength-dispersive (WDS) spectrometer from Oxford Instruments, to obtain information on qualitative and quantitative chemical composition of the microarea of the grain under study [9]. The measurement error was 0,01 %.
For every sample a series of microanalyses was performed, comprising a dozen to several dozen measurements of the chemical composition of characteristic zinc oxide grains, which formed one of the phase components of the samples examined. These measurements enabled determination of the content of dominating chemical components of these grains and the content of accompanying elements in zinc oxide. About 10 chemical composition measurements were made in the microarea of any single grain microarea, and the arithmetic average was taken as the final result. The areas for analysis were selected on the basis of microscope scanning images obtained by detecting secondary electrons as well as backscattered electrons. Images obtained by detecting secondary electrons were used mainly for observing the morphology of dust particles, whereas the signals originating from backscattered electrons enabled, after appropriate processing, obtaining scanning images, the contrast of which depended exclusively on differences in chemical composition, which significantly facilitated the selection of points for analysis.
3. Results
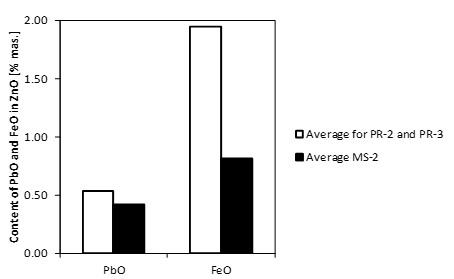
The zinc oxide grains present in the charge mixture (sample MS-2) contain inclusions of lead oxide and iron oxide (Tables 1 and 2). This is evidenced by the chemical composition of zinc oxide grains [4]. It is apparent that in the dust samples (PR-2 and PR-3) iron oxide and lead oxide have a higher share in the ZnO phase composition as compared to the charge mixture samples (MS-2) (Fig. 1).
ZnO contains admixtures of various accompanying elements, such as: Si, Al, Mn, Mg, Ca, K, Ag, As, Cd, Cu, Se, Sb, Sn.
The content of the various accompanying elements in ZnO of the charge mixture sample MS-2 is highly variable, with the highest average value being 0.71 wt.% for arsenic (maximum value exceeding 2 wt.%) and the lowest average value being 0.10 wt.% for potassium (Fig. 2).
Noteworthy is the absence of magnesium in zinc oxide of the charge mixture. The content of any accompanying element in individual ZnO grains of the charge mixture rarely exceeds 0.5 wt.%. In the twelve measurement areas, the content of over 0.5 wt.% was found (Tables 1 and 2):
- in only one area in the case of Si (MS-2-6), Sb (MS-2-10) and Sn ( MS-2-10),
- in two areas in the case of Al (MS-2-7 and MS-2-9) and Se (MS-2-8, MS-2-11),
- in three areas in the case of Cd (MS-2-8, MS-2-10, MS-2-11),
- in five areas in the case of As (for example MS-2-2, MS-2-5, MS-2-7, MS-2-8).
In the remaining measurement areas of sample MS-2 the content of any element did not exceed 0.5 wt.%.
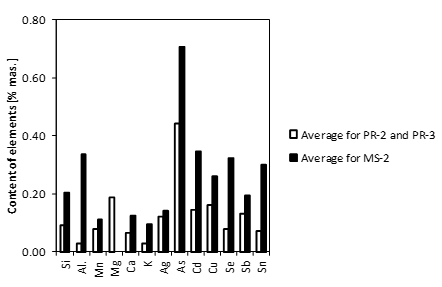
As was the case of the charge mixture, zinc oxide grains present in roasting dust (samples PR-2 and PR-3) contain inclusions of lead oxide and iron oxide (Table 1). The highest concentrations found were those of arsenic (average 0.44 wt.%, maximum content over 1 wt.%). The lowest concentrations found were those of potassium (average 0.03 wt.%). The content of accompanying elements in ZnO, rarely being higher than 0.5 wt.%, is much lower than in the case of grains of this phase in the charge mixture. In the twelve measurement areas, the content of over 0.5 wt.% was found (Tables 1 and 2):
- in only one area in the case of Mg (Pr-3-1), Ca (Pr-2-5, Ag (Pr-2-7), Cd (Pr-2-4) and Sb (Pr-2-1),
- in five areas in the case of As (for example Pr-2-3, Pr-2-7).
In the remaining measurement areas of samples PR-2 and PR-3 the content of any element did not exceed 0.5 wt.%. Noteworthy was the fact that magnesium content in ZnO grains in samples PR-2 and PR-3 was 0.19 wt.% on the average.
Table 1. Content of accompanying elements and phase composition of zinc oxide (PbO, ZnO and FeO) in PR-2 and PR-3 (in wt.%). Explanation: ![]() - average of 12 samples.
- average of 12 samples.
| Element | Pr-2-1 | Pr-2-2 | Pr-2-3 | Pr-2-4 | Pr-2-5 | Pr-2-7 | Pr-3-1 | Pr-3-2 | Pr-3-3 | |
| Si | 0.00 | 0.00 | 0.46 | 0.09 | 0.00 | 0.00 | 0.29 | 0.04 | 0.21 | 0.09 |
| Al | 0.26 | 0.04 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.03 |
| Mn | 0.07 | 0.06 | 0.00 | 0.18 | 0.05 | 0.16 | 0.05 | 0.00 | 0.35 | 0.08 |
| Mg | 0.34 | 0.42 | 0.00 | 0.00 | 0.00 | 0.00 | 1.10 | 0.38 | 0.00 | 0.19 |
| Ca | 0.00 | 0.04 | 0.00 | 0.00 | 0.52 | 0.04 | 0.09 | 0.06 | 0.00 | 0.07 |
| K | 0.01 | 0.05 | 0.00 | 0.00 | 0.00 | 0.14 | 0.04 | 0.00 | 0.00 | 0.03 |
| Ag | 0.13 | 0.20 | 0.14 | 0.14 | 0.00 | 0.61 | 0.00 | 0.00 | 0.14 | 0.12 |
| As | 0.02 | 0.00 | 1.27 | 0.25 | 0.00 | 1.13 | 0.00 | 0.00 | 0.00 | 0.44 |
| Cd | 0.08 | 0.33 | 0.12 | 0.22 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.15 |
| Cu | 0.19 | 0.35 | 0.09 | 0.50 | 0.00 | 0.00 | 0.05 | 0.20 | 0.04 | 0.16 |
| Se | 0.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.49 | 0.10 | 0.08 |
| Sb | 0.00 | 0.57 | 0.00 | 0.00 | 0.00 | 0.23 | 0.07 | 0.30 | 0.25 | 0.13 |
| Sn | 0.00 | 0.48 | 0.00 | 0.06 | 0.13 | 0.00 | 0.00 | 0.00 | 0.06 | 0.07 |
| Content of phases in ZnO | ||||||||||
| PbO | 0.18 | 0.54 | 0.24 | 1.61 | 0.43 | 0.71 | 0.28 | 0 | 0.68 | 0.54 |
| ZnO | 99.45 | 98.74 | 94.59 | 93.6 | 94.32 | 98.54 | 98.52 | 99.55 | 97.94 | 97.52 |
| FeO | 0.37 | 0.72 | 5.17 | 4.79 | 5.25 | 0.75 | 1.19 | 0.45 | 1.38 | 1.95 |
The differentiation between the content of the accompanying elements in ZnO grains of the charge mixture sample MS-2 and dust samples PR-2 and PR-3 cannot be made “directly” due to the presence of inclusions, mainly of lead oxide and iron oxide. These phases may also contain accompanying elements, and the amounts determined are the overall quantities of these elements present in all phases of ZnO grains.
In order to demonstrate the association between the various accompanying elements and the phases present in the examined grains of the charge mixture and of the roasting dust (MS-2, PR-2, PR-3), the values of the correlation coefficient were determined (Tables 3a and 3b).
Table 2. Content of accompanying elements and phase composition of zinc oxide (PbO, ZnO and FeO) in MS-1 (in wt.%). Explanation: ![]() - average of
12 samples.
- average of
12 samples.
| Element | Pr-2-1 | Pr-2-2 | Pr-2-3 | Pr-2-4 | Pr-2-5 | Pr-2-7 | Pr-3-1 | Pr-3-2 | Pr-3-3 | |
| Si | 0.08 | 0.25 | 0.77 | 0.39 | 0.14 | 0.22 | 0.12 | 0.01 | 0.20 | 0.08 |
| Al | 0.31 | 0.05 | 0.10 | 0.65 | 0.18 | 2.03 | 0.15 | 0.11 | 0.34 | 0.31 |
| Mn | 0.12 | 0.00 | 0.00 | 0.16 | 0.24 | 0.01 | 0.08 | 0.31 | 0.11 | 0.12 |
| Mg | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ca | 0.01 | 0.30 | 0.30 | 0.20 | 0.06 | 0.09 | 0.03 | 0.29 | 0.12 | 0.01 |
| K | 0.03 | 0.07 | 0.07 | 0.21 | 0.01 | 0.02 | 0.29 | 0.24 | 0.09 | 0.03 |
| Ag | 0.19 | 0.02 | 0.03 | 0.08 | 0.13 | 0.20 | 0.08 | 0.39 | 0.14 | 0.19 |
| As | 2.13 | 0.90 | 0.04 | 0.56 | 0.94 | 0.40 | 0.25 | 0.06 | 0.71 | 2.13 |
| Cd | 0.27 | 0.43 | 0.37 | 0.34 | 0.65 | 0.04 | 0.53 | 0.91 | 0.35 | 0.27 |
| Cu | 0.26 | 0.36 | 0.22 | 0.07 | 0.27 | 0.25 | 0.27 | 0.43 | 0.26 | 0.26 |
| Se | 0.11 | 0.14 | 0.22 | 0.20 | 0.82 | 0.47 | 0.46 | 0.61 | 0.32 | 0.11 |
| Sb | 0.03 | 0.04 | 0.28 | 0.20 | 0.35 | 0.01 | 0.55 | 0.28 | 0.19 | 0.03 |
| Sn | 0.10 | 0.28 | 0.41 | 0.31 | 0.20 | 0.11 | 0.63 | 0.47 | 0.30 | 0.10 |
| Content of phases in ZnO | ||||||||||
| PbO | 0.63 | 0.01 | 0.17 | 1.05 | 1.39 | 0.16 | 0.37 | 0.11 | 0.42 | 0.63 |
| ZnO | 99.36 | 99.86 | 98.79 | 94.87 | 98.42 | 99.6 | 99.11 | 98.44 | 98.77 | 99.36 |
| FeO | 0.01 | 0.13 | 1.04 | 4.07 | 0.2 | 0.24 | 0.52 | 1.45 | 0.81 | 0.01 |
The values of the correlation coefficients of the various accompanying elements in the ZnO phase and phases that form inclusions within ZnO (lead oxide and iron oxide) indicate explicitly that there are no significant correlations (p<0.05) both in the dust examined (samples PR-2 and PR-3) and in the charge mixture (MS-2). However, the correlation coefficients, despite not being significant, may indicate that there is a tendency for the accompanying elements to accumulate in the various phases of the grains included in ZnO. This in particular applies to those elements that have positive values of correlation coefficients in the case of one particular phase, and negative or close to zero values in the case of the other phases. Such relationship is observed, for instance, in the case of copper. This element has a relatively high (though not significant) positive correlation coefficient for ZnO (ρ=0.57), and a negative (again, not significant) correlation coefficient for PbO (ρ=-0.43) and FeO (ρ=-0.52). This may indicate an association between copper and the ZnO phase. When all the elements determined are reviewed in this aspect, the following tendencies of accumulation of elements may be observed in the various phases:
- in ZnO: (i) in dust – Al, Mg, Se and Sb, (ii) in charge mixture – Ag and Cu,
- in PbO: (i) in dust – Mn, Ag, Cd, Cu and Sn, (ii) in charge mixture – Se and Sb,
- in FeO: (i) in dust – Si, Ca and As, (ii) in charge mixture – Si, Ca, K and Sn.
Table 3a. The values of the correlation coefficient between accompanying elements in zinc oxide and phase components (PbO, ZnO and FeO) thereof in dust samples PR-2, PR-3 and MS-1.
| Samples PR-2 and PR-3 | |||||||||
| Si | Al | Mn | Mg | Ca | K | Ag | As | ||
| Samples MS-1 |
Si | -0.20 | 0.11 | 0.23 | -0.18 | -0.24 | -0.08 | 0.15 | |
| Al | 0.06 | -0.02 | 0.23 | -0.15 | -0.03 | 0.09 | -0.29 | ||
| Mn | -0.49 | -0.26 | -0.19 | -0.13 | 0.07 | 0.41 | -0.29 | ||
| Mg | -0.02 | 0.04 | -0.22 | -0.51 | |||||
| Ca | 0.54 | -0.10 | -0.06 | -0.09 | -0.22 | -0.33 | |||
| K | -0.06 | -0.15 | 0.36 | 0.26 | 0.75 | 0.29 | |||
| Ag | -0.50 | 0.11 | 0.69 | -0.14 | 0.25 | 0.34 | |||
| As | -0.31 | -0.04 | -0.10 | -0.33 | -0.43 | -0.15 | |||
| Cd | -0.11 | -0.39 | 0.64 | 0.45 | 0.57 | 0.27 | -0.28 | ||
| Cu | -0.35 | -0.18 | 0.01 | 0.15 | -0.03 | 0.12 | 0.12 | ||
| Se | -0.27 | 0.17 | 0.59 | -0.04 | 0.21 | 0.39 | -0.27 | ||
| Sb | 0.02 | -0.32 | 0.43 | -0.07 | 0.66 | 0.25 | -0.44 | ||
| Sn | 0.13 | -0.42 | 0.12 | 0.22 | 0.74 | -0.01 | -0.70 | ||
| PbO | -0.02 | -0.01 | 0.38 | -0.21 | -0.07 | -0.23 | 0.34 | ||
| ZnO | -0.28 | -0.05 | -0.35 | -0.27 | -0.42 | 0.11 | 0.11 | ||
| FeO | 0.34 | 0.06 | 0.27 | 0.40 | 0.54 | -0.03 | -0.27 | ||
Table 3b. Continuation of Table 3a.
| Samples PR-2 and PR-3 | |||||||||
| Cd | Cu | Se | Sb | Sn | PbO | ZnO | FeO | ||
| Samples MS-1 |
Si | -0.17 | -0.21 | -0.17 | -0.20 | -0.27 | -0.12 | -0.35 | 0.42 |
| Al | 0.02 | 0.06 | 0.49 | -0.11 | -0.05 | -0.23 | 0.34 | -0.32 | |
| Mn | 0.26 | -0.06 | -0.08 | 0.20 | 0.01 | 0.42 | -0.13 | 0.03 | |
| Mg | -0.15 | -0.04 | 0.21 | 0.21 | 0.08 | -0.32 | 0.38 | -0.34 | |
| Ca | -0.14 | -0.37 | -0.13 | -0.14 | 0.16 | -0.07 | -0.40 | 0.46 | |
| K | 0.75 | -0.38 | -0.27 | 0.38 | 0.19 | 0.35 | 0.22 | -0.33 | |
| Ag | 0.96 | -0.16 | -0.17 | 0.33 | 0.07 | 0.27 | 0.04 | -0.12 | |
| As | 0.35 | -0.12 | -0.43 | -0.32 | -0.34 | 0.00 | -0.10 | 0.11 | |
| Cd | -0.06 | -0.21 | 0.33 | 0.09 | 0.21 | 0.05 | -0.11 | ||
| Cu | 0.35 | 0.05 | 0.04 | 0.22 | 0.15 | -0.02 | -0.02 | ||
| Se | 0.62 | 0.21 | 0.14 | -0.25 | -0.41 | 0.44 | -0.38 | ||
| Sb | 0.56 | -0.10 | 0.53 | 0.72 | 0.03 | 0.39 | -0.45 | ||
| Sn | 0.51 | 0.17 | 0.14 | 0.67 | 0.25 | -0.03 | -0.04 | ||
| PbO | 0.18 | -0.43 | 0.35 | 0.13 | -0.30 | -0.51 | 0.30 | ||
| ZnO | -0.20 | 0.57 | -0.04 | -0.16 | -0.07 | -0.61 | -0.97 | ||
| FeO | 0.17 | -0.52 | -0.09 | 0.14 | 0.21 | 0.34 | -0.95 | ||
There is also a group of elements the correlation coefficients of which in relation to one particular phase are negative, and those in relation to the remaining phases are positive (sometimes the values being similar). This may indicate a tendency of some elements to accumulate in two phases. Thus:
- accumulation of K in PbO and ZnO phases in dust,
- accumulation of As in PbO and ZnO phases, and Mn, Cd and Sb in PbO and FeO phases in charge mixture.
There is also a significant correlation between some accompanying elements. And so we have:
- in dust significant correlations were observed between: Ag and K (ρ=0.75), Cd and K (ρ=0.75), Cd and Ag (ρ=0.96), Sn and Sb (ρ=0.72),
- in the charge mixture significant correlations were observed between: Ag and Mn (ρ=0.69), Cd and Mn (ρ=0.64), Se and Mn (ρ=0.59), Se and Cd (ρ=0.62), Sb and K (ρ=0.66), Sn and K (ρ=0.74), Sn and As (ρ=-0.70), Sn and Sb (ρ=0.67).
4. Conclusions
Zinc oxide grains, both in the charge mixture (MS-2) and in roasting dust (PR-2 and PR-3), contain inclusions of lead oxide and of iron oxide. The presence of the following accompanying elements in the grains was established: Si, Al, Mn, Mg, Ca, K, Ag, As, Cd, Cu, Se, Sb, Sn. The content of these elements in ZnO grains shows large variation, whereas in both the charge mixture and the roasting dust the highest concentration was that of arsenic: 0,71 wt. % in the charge mixture, and 0.44 wt.% in the dust.
Comparison of the concentrations of particular accompanying elements in zinc oxide grains in the charge mixture and in the roasting dust shows the absence of magnesium in ZnO in sample MS-2 and the presence of that element in samples PR-2 and PR-3 at an average concentration of 0.19 wt.%.
Accompanying elements show low values of the correlation coefficient with zinc oxide and with identified phases (FeO and PbO) that form inclusions in ZnO grains. However, the correlation coefficients, despite not being statistically significant, may indicate that there is a tendency for some accompanying elements to accumulate both in ZnO grains, as well as in FeO or PbO grains. Correlation coefficients may indicate the following accumulation tendencies: of Ag, Cu in ZnO in the charge mixture, and of Al, Mg, Se and Sb in ZnO in dust; of Se, Sb in PbO in the charge mixture, and Mn, Ag, Cd, Cu and Sn in dust; of Si, Ca, K and Sn in FeO in the charge mixture, and Si, Ca and As in dust.
At the same time some of the accompanying elements (K, As, Mn, Cd and Sb ) have negative correlation coefficients in relation to one phase, and positive in relation to the other phases. For this reason it is difficult to indicate which phase is the main carrier of a particular element.
Some of the accompanying elements present in zinc oxide grains show significant correlations in relation to each other: Ag and K, Cd and K, Cd and Ag, Sn and Sb in the charge mixture, and Ag and Mn, Cd and Mn, Se and Mn, Se and Cd, Sb and K, Sn and K, Sn and As, Sn and Sb in dust.
References
[1] Zinc and Lead Production Process in “Miasteczko Śląskie” Zinc Smelting Plant. Technical documentation, 2000.
[2] M. A. Barakat, “Pyrometallurgical processing of zinc ash and flue dust,” Acta Montanistica Slovaca, vol. 9, no. 4, pp. 259-269, 2003.
[3] M. Pozzi, K. Nowińska, “Distribution of Selected Accompanying Elements in Zn-Pb Concentrates in the Imperial Smelting Process,” (in Polish), Wydawnictwo Politechniki Śląskiej, 2006.
[4] Z. Adamczyk, E. Melaniuk-Wolny, K. Nowińska, “The mineralogical and chemical study of feedstock mixtures and by-products from pyrometallurgical process of zinc and lead production,” Wydawnictwo Politechniki Śląskiej, 2010.
[5] Z. Adamczyk, K. Nowińska, “Skład chemiczny pirytu w mieszance wsadowej do pirometalurgicznego procesu otrzymywania cynku i ołowiu,” Systemy Wspomagania Inżynierii Produkcji, vol. 5, no. 17, pp. 38-47, 2016. View Article
[6] J. Ruetten, “The Waelz Process for the Treatment of EAF Dust 2004 - 2008 – 2012,” presented at GDMB - Zinc Experts Meeting, Kokkola, ValoRes GmbH, 2009.
[7] Z. Adamczyk, K. Nowińska, E. Melaniuk-Wolny, “Variation of the content of accompanying elements in galena in pyrometallurgical process of zinc and lead production,” Acta Montanistica Slovaca, vol. 1, no. 3, pp. 158-163, 2013. View Article
[8] K. Nowińska, Z. Adamczyk, E. Melaniuk-Wolny, “Accompanying elements in sphalerite in pyrometallurgical process of zinc and lead production,” Materials and Manufacturing Processes. vol. 30, no. 12, pp. 1457-1464, 2015. View Article
[9] J. Sokołowski, M. Nosiła, B. Pluta, “Fundamentals of X-Ray Microanalysis,” (in Polish), Wydawnictwo Politechniki Śląskiej.

